Lubricants from ADDINOL
The lubricants of ADDINOL meet the highest demands on different requirements. We offer a wide range of products for industry and automotive. You are welcome to delve deeper into the individual topics or search for the suitable lubricant with our Oilfinder.
If you would like to find out more about lubricants in general, you can inform yourself on this page about aspects such as composition, properties or tasks.
Information about lubricants
Tasks | Production | Properties | Composition | Viscosity | Deterioration | DisposalTasks of lubricants
Lubricants of any kind are intended to reduce friction in a mechanical process and to separate two touching bodies as best as possible. This ensures a good work process and reduces unpleasant side effects such as wear or heat. Optimally, all lubrication points of a machine are recorded and defined by a lubrication plan.
If two objects meet, occasionally the smallest bumps in the material form, which cause more and more resistance and wear between the friction partners. Lubricants slow this process significantly and ensure a longer life of e.g. engines, transmissions or hydraulics.
In summary, the most important functions of lubricants are:
- Lubricate: reduce friction, reduce wear (e.g. abrasive and adhesive wear)
- Cooling: dissipation of the frictional heat of the sliding partners
- Protect: protect material against corrosion
- Keeping clean: remove dirt particles and combustion residues to the filter
- Transport: deliver additives to the material
- Sealing: tight sealing at critical points
- Transferring forces: in power steering, hydraulics, etc.

Typical lubrication methods include splash lubrication and pressure lubrication.
Production of lubricants
The base oils for the lubricant production are extracted from crude oil. The oil consists of hydrocarbon compounds, which can be broken down into individual components by chemical processes. By means of atmospheric distillation, various end products are obtained from the crude oil, which split off in certain temperature ranges. By warming, evaporating and condensing, the oil separates into its natural components. These include natural gas, gasoline, diesel, lubricating oils and tar. Gases such as methane consist of simple hydrocarbon compounds such as CH4. Gasoline is much more complex and consists of 5-12 carbon atoms. Lubricating oils are made of 20-35 carbon atoms. Bitumen consitst of over 80 linked carbon atoms.
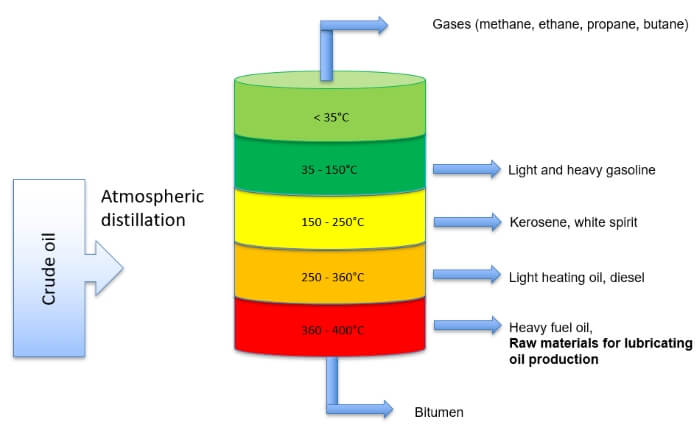
The mineral oil raffinate must be refined again after extraction to form a higher quality base product. The purer the carbon-hydrogen compound is, the more efficient is the oil in the application. This improves the aging resistance, the viscosity-temperature behavior and the cold start behavior of the lubricating oil.
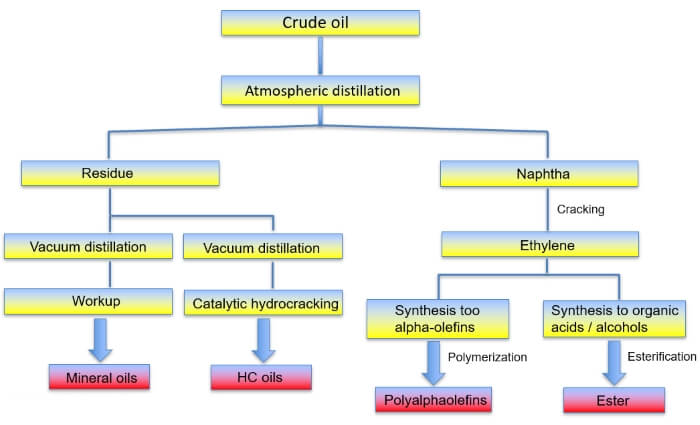
Special processes such as cracking or hydrocracking convert less valuable constituents of the crude oil into valuable lubricants. Cracking breaks up hydrocarbon molecules of length C5 to C12 and reduces them to the size of gases (C2 and C3). This produces synthetic hydrocarbons such as PAO, PIB or PIO. Hydrocracking describes the process of decomposing long-chain hydrocarbon compounds (>C35) to oil molecule size (C20 to C35). Hydrogen atoms attach themselves to the fractured points and close the gaps in the molecular structure.

Lubricant properties
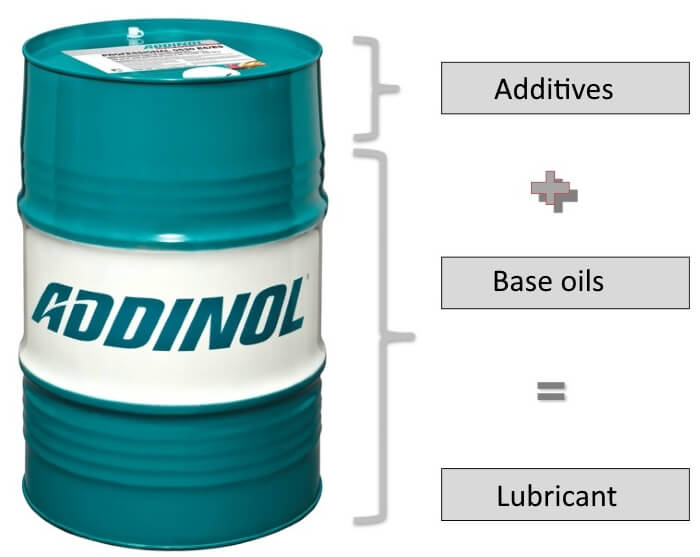
Lubricants consist of base oils and additives, which are mixed differently depending on the application. For example, oils for cars with combustion engines have to meet completely different requirements than oils for cars with electric engines. In the case of base oils, viscosity, degree of refinement and pre-treatment are essential quality characteristics. As already described, a distinction is made between mineral oils (extracted from crude oil) and synthetic oils (artificial oil production). In development, the automotive industry is striving to reduce emissions, reduce consumption, extend oil change intervals and extend the service life of lubricants. This is why more efficient base oils are required, which must have properties such as low sulphur content, high thermal stability or high homogeneity.
Composition of lubricants
Additives are added to the base oils, which have a decisive influence on the subsequent performance of the end product with the best possible quality. Additives improve or strengthen the properties of the base oil and also add new properties to the base oil.
Frequently used additives in lubricants are:
- Corrosion protection additives
- Oxidation inhibitors
- Wear protection additives
- Detergents/Dispersants
- VI improver
Lubricants are therefore always composed of pure base oils and specially added additives, which are selected according to the area of application of the oil. The main component of a lubricant is always the base oil. The additives take up about 10-20% of the lubricant. In exceptional cases, the additive content can reach up to 30%.
Viscosity of lubricants
The viscosity or toughness is a measure of the internal friction of an oil when flowing. It is strongly temperature-dependent:
- Cold oils have a high internal friction and are therefore highly viscous
- Warm oils have less internal friction and are low viscous
The viscosity thus refers to the thinness or thickness of a lubricant. However, viscosity is never a quality feature of an oil. Depending on the application, lubricants with low or high viscosity are required.

Deterioration of the lubricants
Due to constant load, natural ageing and other influences, the lubricants eventually wear out.
The most common reasons for a deterioration of the lubricants are:
- Viscosity change due to heat / cold
- Chemical contamination by acids
- Oxidation
- Gas or water ingress
- Pollution by particles
- Degradation of additives and loss of oil properties
Then it is time to make an oil change, in order not to risk damage to the friction partners and continue to ensure a good lubrication of the components. In most cases, the manufacturer of the machines determines oil change intervals. You can test the used oil in the lab for its properties to set the time for a change, too.

Correct disposal of lubricants
If lubricants do not work as desired anymore, the challenge is to dispose the used oil properly. Under no circumstances may you dump the oil in the normal household waste or pour it into the sink. Even the smallest amounts of leaked oil can contaminate thousands of liters of groundwater. Anyone who gets caught in the pollution, must expect high fines.
The disposal of used oil is safer and more environmentally friendly in the following ways:
- Dispose of the oil at the dealer: The dealer is obliged to take back the used oil free of charge for recycling or disposal.
- Garbage collection at the municipality: Each municipality has a recycling center or collection point where old lubricants can be returned. For larger amounts of waste oil costs may arise.
- Used oil disposal at the filling station: Petrol stations usually have collecting tanks or suction devices in which the oil can be filled. You should inform yourself before disposing of the waste oil, if there are any costs.
- Oil disposal at the workshop: If the workshop carries out an oil change, it usually disposes of the used oil automatically.
Before disposing of the lubricant, make sure that it is not contaminated by other substances. In addition, various waste oils should not be poured together, but disposed of separately.
Contact

Find a sales partner
Are you interested in our lubricants? Find a sales partner near you.